Geographical Distribution in Africa
The African bollworm is reported to be present in all African countries (CABI Compendium 2006). Updated on 10 July 2019. Source CABI
General Information on Pest and Damage
Introduction
The African bollworm is a pest of major importance in most areas where it occurs. It damages a wide variety of food, fibre, oilseed, fodder and horticultural crops. It is a major pest due to its high mobility, its ability to feed on many species of plants, its high fecundity and reproductive rate, and its capacity to develop resistance to pesticides. The habit of feeding inside the fruiting parts of the plant during most of its development makes bollworms less vulnerable to insecticides. Pesticides should be applied before the caterpillars bore into the fruits/pods. The African bollworm has a strong ability to develop resistance to insecticides. Currently there is a widespread occurrence of resistance in bollworms to popular synthetic pyrethroids in Africa and elsewhere.
Damage
The severity of the damage varies between crops, regions and locations, and between seasons.
Caterpillars of the African bollworm feed on leaves, buds, growing points, flowers and fruit. Leaf damage reduces leaf area, which can slow plant growth. Feeding on flowers and fruit causes the main damage. Flower feeding can prevent fruit formation. Caterpillars usually bore clean, circular holes through fruits/pods. Excrements (faeces / waste) of the feeding caterpillars are placed away from damaged plant parts. The holes serve as entry points for secondary infection by diseases causing fruit decay. One caterpillar can damage several fruits/pods. Once they burrow into the fruits/pods they are difficult to reach and to control with insecticides. Often caterpillars feed with the head and forepart of the body inside the fruit/pod and the rest of the body outside.
Moreover, its preference for the harvestable flowering parts of high-value crops including cotton, tomato, sweet corn, and cut-flowers makes it responsible for huge economic losses and socio-economic costs. Crop losses at farm level in Kenya has been estimated at over 50% on cotton and pigeon pea, over 20% on sorghum and millet, and over 2 million stems on cut flowers (Kibata, 2002). In addition, the African bollworm is a quarantine pest. This is important for export crops. If a caterpillar of this pest is detected in a consignment of an export commodity (e.g. flowers, vegetables, etc) shipped to Europe, the whole consignment is rejected.
Host range
The African bollworm has been reported on 35 crops and 25 wild host plants in eastern and southern Africa (Greathead and Girling 1989). The severity of the damage varies between crops, regions and locations, and between seasons. In eastern Africa, attacked crops include cotton, French beans, dry beans, okra, peas, legumes, maize, sorghum, sunflower, tobacco and tomato. Among these crops, the African bollworm is considered a key pest of cotton, chickpea, pigeon pea and tomato (Sithanantham et al., 2002). In South Africa crops attacked include peas, beans, wheat, cotton, maize, grain sorghum, oats, barley, sunflower, tobacco, citrus, cucurbits, potato, tomato, lucerne, sunnhemp, cape gooseberry, chickpea and groundnuts (Cherry et al., 2003). Amaranthus spp., Cleome sp. and Acalypha sp. are important wild host plants in Africa.
Symptoms
Larvae feed on leaves, flower buds, flowers, grains, and bore into pods and fruits. Excrements (faeces / waste) of the feeding caterpillars are evident on damaged plant parts.
Affected plant stages
Vegetative growing stage, flowering stage and fruiting stage.
Affected plant parts
Leaves, growing points, inflorescence and fruits/pods.
Biology and Ecology of the African Bollworm
 |
| African bollworm eggs are tiny (diameter 0.4-0.6 mm), yellowish-white and glistening at first, changing to dark-brown before hatching; pomegranate-shaped. |
|
© A.M. Varela, icipe
|
The eggs are tiny (about 0.5 mm in diameter), round and yellowish-white in colour. They darken before hatching. They are deposited singly on tender parts of the plant. Egg-laying generally coincides with early flowering of host crops.
 |
| African bollworm on French beans. Fully grown caterpillars are 3-4 cm long. |
|
© A.M. Varela, icipe
|
Young caterpillars (larvae) are generally yellowish-white to reddish brown. They have a dark brown to black head and several rows of black bumps with short hairs along their backs, which give them a spotted appearance. Fully-grown caterpillars are 35 - 40 mm long. Older caterpillars vary in colour from almost black, brown or green to pale yellow with dark grey yellow stripes along the sides of the body. However, all caterpillars have a typical light stripe along each side of the body. The head is brown or green and mottled. The fully-grown caterpillars drop from the plant and burrow into the soil to pupate.
 |
| The African bollworm pupa is shiny brown, about 16 mm long |
|
© A.M. Varela, icipe
|
The pupa is shiny brown, about 16 mm long, with smooth surface, with two short parallel spines at the posterior tip of the body. Pupation takes place in the soil.
 |
| Adult moths of African bollworm are about 14 to 18 mm long with a wingspan of 35 to 40 mm. |
|
© A.M. Varela, icipe
|
The adult moth is fleshy, yellowish-brown with a dark speck, greyish irregular lines and a black kidney-shaped mark on the forewings. The hindwings are whitish with a black patch along the outer margin. The moth is about 14 to 18 mm long with a wingspan of 35 to 40 mm. They are relatively strong fliers, dispersing widely within areas where the host plants are found. They can also be carried by strong winds. Moths are strongly attracted to plants that provide honeydew or nectar.
The life cycle of African bollworm
Moths lay a large number of eggs, and the life cycle may be completed in a short time under warm conditions. Eggs hatch in 3 to 5 days. Larval and pupal periods last 17 to 35 and 17 to 20 days, respectively. The life cycle is completed in 25 to 60 days depending on temperature.
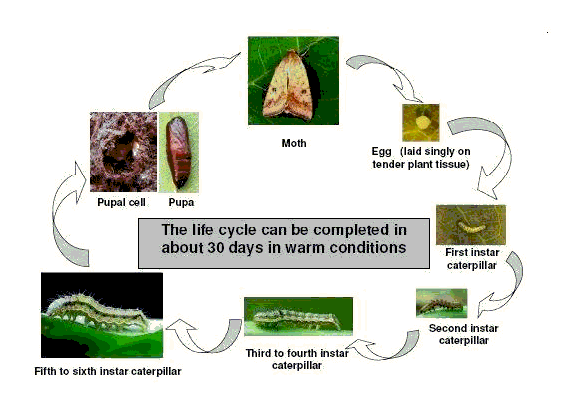 |
| Life cycle of African bollworm |
|
© A.M. Varela, icipe
|
Pest and disease management
Pest and disease Management: General illustration of the concept of infonet-biovision
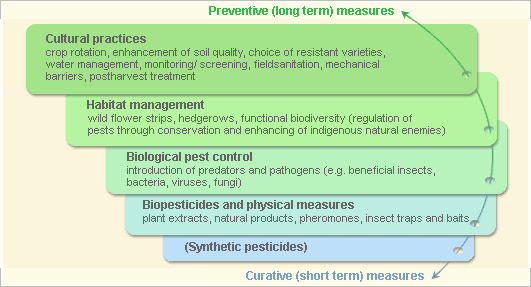
This illustration shows the methods promoted on infonet-biovision. The methods shown at the top have a long-term effect, while methods shown at the bottom have a short-term effect. In organic farming systems, methods with a long-term effect are the basis of crop production and should be of preference. On the other hand methods with a short-term effect should be used in emergencies only. On infonet we do not promote synthetic pesticides.
Further below you find concrete preventive and curative methods against African bollworm
Cultural practices
Monitoring and Decision Making
Look out for eggs and small caterpillars. Early detection of eggs, or young caterpillars before they bore into the fruits or pods is very important. Once the caterpillars have entered the fruit/pod they are difficult to control and by then they have caused damage. Early detection can be achieved by regular scouting of the crop. Monitoring moth population by using pheromone traps reduces crop inspection time considerably and leads to timely intervention. Check also for natural enemies. Parasitised eggs are easy to recognise. Healthy eggs are whitish yellow in colour, and they turn black when parasitised.
Action thresholds have been developed for high value crops (e.g. tomato, cotton, pulses and tobacco). In these crops the tolerance for insect damage is low and therefore, economic damage thresholds are low. The following thresholds are given as examples.
Cotton: In Malawi and Zimbabwe, thresholds based on egg numbers have been used successfully in cotton since 1961. Spraying was recommended at an average of one egg per two plants in twice-weekly counts. In the Sudan Gezira, over two eggs or caterpillar per 18 plants, and in Australia two eggs per metre of row were used as thresholds (CABI, 2004). It has been argued that control thresholds based on damage are easier to use and more economical than those based on pest density. In the case of cotton, damaged buds are easier to detect and sample than either eggs or small caterpillars. Studies in Tanzania indicated that spraying at damage threshold of 10 to 20% would give adequate protection to the crop. Further fine-tuning of damage thresholds should be concentrated during the first four weeks of flowering when most of the damage by this pest occurs. (Kabissa, 1989).
Tomato: It has been recommended to randomly select 30 tomato plants and examine the leaves immediately below the topmost open flowers to look for eggs of African bollworm (AVRDC, 2000).
The decision to make an intervention to manage this pest should be based on an analysis of the situation (stage of the crop, presence of natural enemies and economic return (based on market value of the crop and the value of the intervention).
Sanitation (Clean cultivation)
- Remove and destroy plant residues immediately after harvesting.
- Plough the soil after harvesting. This exposes pupae, which may then be killed by natural enemies or through desiccation by the sun.
Mechanical control
- Handpick and destroy eggs and small caterpillars. This is feasible in small plots or when infestations are low. For instance, it has been reported that in Ethiopia when pest damage in sorghum is minor, farmers shake the plant to induce the caterpillar to drop from the sorghum head and pick them by hand (Negash and Abate, 2002). It is very important to detect small caterpillars before they enter the fruits.
- If African bollworms are detected in the field, sort out the harvested crop very thoroughly and remove the caterpillars manually. This is particulary important for export crops to minimise/avoid rejection in the importing country.
Habitat Management
Intercropping and trap crops
Moths of the African bollworm prefer to lay eggs on certain crops (e.g. pigeon pea, chick pea, crotalaria, maize, tobacco, African marigold, sorghum and sunflower), especially during the flowering period. These crops may be utilised to distract moths from crops that are more vulnerable to African bollworm damage. If trap crops are planted in strips or around the field, the moths will lay eggs on them instead of the main crop. A careful choice of the trap crops and planting dates might ensure the maximum effectiveness of trap crops. Heavy infestations of the African bollworm on the trap crops need to be controlled to prevent build-up of the pest populations.
Trap cropping and planting diversionary hosts have been widely applied, although with variable results. Some examples are given below:
- Intercropping of cotton with chickpea, cowpea, onion, pearl millet, crotalaria, pigeon pea and marigold in strips is reported to divert the populations of sucking pests and the African bollworm from cotton (Dejen and Tesfaye, 2002).
- It has been shown that flowering sunflower, sorghum and maize are much more attractive to African bollworm moths for egg-laying than cotton. For this reason, maize has been commonly used as a trap crop. Preference for maize has been observed to be so strong that cotton plots remained almost clear of eggs when bordered with a few rows of maize. However, this attractiveness appears to be inconsistent. This may be due to differences between varieties of the host plant or differences between African bollworm populations (van den Berg, 1993).
- Studies of this pest and its natural enemies in cotton, sunflower, maize and sorghum in Kenya showed that survival of the African bollworm is low in maize and sorghum. Therefore, it is suggested that they could be used to divert African bollworm infestation. The selection of the variety is important. In the case of sorghum, studies in Tanzania showed that mortality of the African bollworm was high in sorghum varieties with open panicles, which give little protection from natural enemies. In contrast, pest mortality in compact head sorghum varieties with dense and semi dense panicles was low. Cotton grown adjacent to compact head varieties suffered a rapid build-up of the African bollworm as the sorghum crop reached maturity (Nyambo, 1989). Moreover, when using maize or sorghum as trap crops to protect cotton, these trap crops would have to be planted at regular intervals since there is only a short period (flowering) when maize and sorghum are attractive to the moths, whilst on cotton, moths lay eggs over a extended period of time (three months in Kenya) (van den Berg, 1993). This would be feasible if irrigation is available but impractical for rainfed crops.
- Castor, sunflower, black gram, and cowpea are also recommended as traps crops for cotton. These plants attract bollworms as well as provide habitat for natural enemies, which feed on bollworms. It has been recommended to grow a row of castor as border crop, or to sow the other crops in every five rows of cotton (CIKS, 2000).
- Research in Ethiopia indicated that lupin, pigeon pea, hyacinth bean, maize and sunflower attracted significantly higher numbers of this pest and diverted them from the main crop haricot beans (Dejen and Tesfaye, 2002). It has been reported that maize grown as a strip crop at 10 m intervals reduced pod damage by the African bollworm in haricot beans (Ahmed and Damte, 2002).
- Intercropping chickpeas with wheat, barley, linseed, mustard and safflower are reported to reduce attack by the African bollworm on chickpeas in India. On the other hand, intercropping chickpeas with lentils and field peas have led to a higher pest infestation in chickpeas (Negash and Abate, 2002)
- African marigold has been used as trap crop in tomato. Marigold planted after every eight rows of tomato helps attract most of the African bollworms moths to marigold (Negash and Abate, 2002).
The following procedure has been recommended to minimise infestation of the African bollworm on vegetable crops in Ghana (Youdeowei, 2002):
- Obtain a suitable trap crop to plant with the main crop
- Plant the trap crop around the vegetable field in strips 10 to 15 cm apart; pigeon peas can be planted as a hedge around the main crop
- Plant the trap crop so that it starts flowering earlier than the main crop and remains flowering thorough the development cycle of the main crop. This way, the bollworms will lay eggs and thrive only on the trap crops
- Regularly observe the populations of bollworms on the trap crop and, if necessary, spray them with a suitable pesticide to control them.
Crop rotation
Avoiding planting crops after each other that are susceptible to bollworm like cotton, maize, sorghum, tobacco, soybean, and tomato may help to reduce/prevent build up of bollworm populations. However, to be effective crop rotation should be done over large areas, since the moths can fly long distances (Dobson et al. 2002). Some crops not susceptible to bollworm that could be planted in rotation with susceptible crops are small grains like rice, and plants of the onion family.
In countries with two distinct seasons (wet and dry), it has been recommended to plant rice followed by beans during the rainy season, and cotton or small grains in the second cropping (Hasse, 1986,1987).
However, it should be noted that the African bollworm also attacks many other plants commonly found in the field (e.g. weeds), for instance Cleome in Tanzania. Therefore, crop rotation alone is not likely to be effective in managing this pest.
Biological pest control
Natural enemies
In Africa, a wide variety of natural enemies have been recorded attacking the African bollworm. The most important are egg parasitoids (e.g. Trichogramma spp.), larval parasitoids (wasps and flies that parasitise caterpillars), and predators such as ants, assassin bugs, minute pirate (anthocorid) bugs, lacewings and ladybird beetles. Over 170 parasitoids and a large number of predators have been reviewed and catalogued, most of them from southern and East Africa (Cherry et al, 2003). Conserving and encouraging these natural enemies should be part of any strategy to manage this pest.
The distribution and importance of natural enemies vary within and between region, between crops and seasons. For example parasitism of eggs by Trichogramma wasps is higher and by more species on sorghum than on other crops. Studies in Kenya showed that predators, mainly anthocorid bugs and ants, were the most important natural enemies of the African bollworm on sunflower, maize, sorghum and cotton (van den Berg, 1993; Cherry et al. 2003), while in northern Tanzania, parasitism and diseases were the major cause of mortality on sorghum, cotton and a weed (Cleome sp.) (Nyambo, 1986, 1990; van den Berg, 1993).
Spraying practices can harm natural enemies. This is particularly detrimental early in the season since natural enemies that may otherwise have built up and suppressed the pest are killed. Substitution of broad spectrum pesticides with selective biopesticides such Bt, nuclear polyhedrosis viruses (NPVs) and botanicals (e.g. neem), for control of this and other pests may permit early establishment of natural enemies and contribute to the control of pests. To achieve better results this should be a joint effort involving neighbours and farmer communities in a regional scale.
Plant crops that are attractive to natural enemies
The impact of natural enemies can be improved through intercropping or adjacent planting of crops that are attractive to them. In cotton, the African bollworm infests the crop before flowering; this is earlier than the immigration of anthocorid bugs into the fields. It is suggested that if crops with distinct flowering periods that attract African bollworm moths and anthocorid bugs at the same time, are planted adjacent or intercropped with young cotton, they would attract anthocorid bugs and other predators early in the season, and at the same time distract moths from cotton. Sorghum seems to be a better 'natural control' plant than maize because it is equally or more attractive to African bollworm, and attracts more anthocorid bugs during flowering. Moreover, sorghum varieties are better adapted than maize to the dry climatic conditions where cotton is commonly grown (van den Berg, 1993).
Ants are important predators of the African bollworm. Ant activity on the crops can be encouraged by changing crop composition, by weed management and by provision of alternative food sources. Plants that offer alternative food sources on the canopy, such as plant exudates or honeydew producing insects, are more attractive to ants. Ants could also be attracted to crops by providing crushed sugar cane, or sprayed sugar solution. Ants would then complete their diet with protein by preying on insects, including caterpillars of the African bollworm. A good example is the BioRe Project in Tanzania, where sunflower is used as a trap crop in and around organic cotton fields. Cannibalism and predation by ants (in particular the bigheaded ants Pheidole spp.) on sunflowers causes high mortality among bollworm caterpillars (Cherry et al, 2003).
Chicken can help by eating caterpillars and pupae at certain time of crop development in small fields. They can be allowed to roam freely on lands not yet planted or where hardy crops are growing. However, they should not be allowed near seedlings or plants with fruit since they may cause damage by scratching and pecking (Dobson et al, 2002; Elwell and Maas, 1995).
Birds that eat pests can be encouraged to visit crop fields. Some changes will encourage them to nest and stay in the area, and this can lead to a permanent increase in local predatory bird populations. For example, groundnut plants are close to the ground and birds cannot use them as vantage points for spotting insect prey. From a perch, however, birds can easily identify prey and swoop down to devour them. Bird perches are resting places for predatory birds to rest and to look for preys such as insect pests of cotton, peanuts, and cowpeas. Predatory birds prefer to look for prey in field crops where they have places to rest. To make bird perches, use bamboo or wooden poles or tree branches. Erect either of these at regular intervals in the field.
To have live bird perches within the field, plant Setaria species (foxtail cultivars). These plants have been found to be attractive to predatory birds. Much larger populations of birds (mynas, finches and blackjays) were recorded in fields with bird perches, and these contributed to a significant suppression of the insect pests. In cotton field, plant Setaria in every 9th or 10th row of cotton. Once the birds are on the fields, they prey on cotton bollworms and other insects (NCIPM, 2000).
For more information on natural enemies click here
Biopesticides and physical methods
Botanicals
Several plants are reported to be useful in the management of the African bollworm on several crops. Garlic is reported to be effective against African bollworm on cotton and maize. For information on how to make your own biopesticide with garlic click here.
Experiments for screening effective botanicals for control of tomato pests, in Western Kenya, indicated that pepper and marigold were effective against pests of tomatoes including African bollworm (Magenya, 2002).
Neem (Azadirachta indica)
There are several reports of use of neem products for control of the African bollworm on several crops. It is important to control the small caterpillar before they enter the fruit, as any later treatment would be ineffective. For more information on how to make your own biopesticide with neem click here.
Bt (Bacillus thuringiensis)
Bt is widely used in Africa for control of caterpillars, including the African bollworm. In particular B.t. subspecies kurstaki and B.t. aizawai control this pest (Cherry et al, 2003; Kibata, 2002). Commercial Bt products for control of caterpillars currently available in Kenya include Dipel®, Thuricide HP® (both Bt var. kurstaki), Florbac 70 DG® and XenTari® (both B.t. var. aizawai). For more information on Bt click here.
Farmer experience
In Kenya, farmers at the Coast use extracts from neem leaves and seeds, and commercial neem products on vegetable crops. They also use extracts from hot pepper or mixtures of plant extracts such as Lantana, chillies and Tephrosia (Kega, 2002; Pole and Kimani, 2002).
Physical methods
- Handpick damaged fruits and collect those that fall down. Destroy the damaged fruits by cutting into small pieces, or place them in sealed sacks and dry under the sun. Do not put them immediately in a compost pit or burying them will enable the matured caterpillars to pupate into the soil.
- Handpick and destroy eggs and caterpillars. It is very important to detect small caterpillars before they enter the fruits. This is feasible at low infestations and in small plots.
- Weed if necessary. Destruction of weeds that may harbour caterpillars is important to prevent African bollworm infestation.
- Plough the field to expose the pupae to predators and the sun.
Information Source Links
- AVRDC. Tomato fruitworm. www.avrdc.org
- Ahmed, S. and Damte, T. (2002). African bollworm: A key pest in pulses production in Ethiopia. In African Bollworm Management in Ethiopia. Status and needs. Proceedings of the National Workshop held at the Plant Protection Research Centre Ambo, Ethiopia. April 2002. pp 27-36.
- Bessin, R. Tomato IPM guidelines. University of Kentucky Entomology. www.ca.uky.edu
- CABI. (2004). Crop Protection Compendium, 2004 Edition. © CAB International Publishing. Wallingford, UK. www.cabi.org
- CIKS. (2000). Bollworm control in cotton. Center for Indian Knowledge Systems. Chennai, India. Pesticide Post. Vol. 8, No 6.
- Cherry, A., Cock, M, van den Berg, and Kfir, R (2003). Biological control of Helicoverpa armiguera in Africa. In Biological Control in IPM Systems in Africa. Neuenschwander, P., Borgemeister, C and Langewald. J. (Editors). CABI Publishing in association with the ACP-EU Technical Centre for Agricultural and Rural Cooperation (CTA) and the Swiss Agency for Development and Cooperation (SDC). pp. 329-346. ISBN: 0-85199-639-6.
- Dejen, A. and Tesfaye (2002). Cultural control of the African bollworm, Heliothis armiguera (Hb.). In African Bollworm Management in Ethiopia. Status and needs. Proceedings of the National Workshop held at the Plant Protection Research Centre Ambo, Ethiopia. April 2002. pp 99-105.
- Dobson, H., Cooper, J., Manyangariwa, W., Karuma, J. and Chiimba, W. (2002). Integrated Vegetable Pest Management- Safe and Sustainable Protection of Small-Scale Brassicas and Tomatoes. Natural Resources Institute, University of Greenwich. 179 pp. ISBN: 0-85954-536-9
- .Elwell, H and Maas, A. (1995). Natural Pest & Disease Control. Natural Farming network, Zimbabwe. The Plant Protection Improvement Programme and the Natural Farming Network.
- Greathead, D.J. and Girling, D. J. (1989). Distribution and economic importance of Heliothis and of their natural enemies and host plants in southern and eastern Africa. In King, E. C. and Jackson, R. D. (eds)
- Proceedings of the Workshop on Biological Control of Heliothis: Increasing the Effectiveness of Natural Enemies. November 11-15 1985. New Delhi, India. Far Eastern Regional Research Office, United States Department of Agriculture. New Delhi, India. pp 329-345.
- Haggis MJ, (1982). Distribution of Heliothis armigera eggs on cotton in the Sudan Gezira: spatial and temporal changes and their possible relation to weather. Proceedings of the International Workshop on Heliothis Management. ICRISAT Center, Patancheru, India, 15-20 November 1981, 87-99; [5 fig.].
- Hasse, V. (1986): Introducing plant protection to cotton farmers in the Philippines. Second International Conference on Plant Protection in the Tropics. Malaysian Plant Protection Society, Kuala Lumpur.
- ICIPE (2003). A Guides to IPM in Brassicas/Tomato Production www.icipe.org
- ICIPE. (2003). Development of biocontrol-based management of Helicoverpa armigera in eastern and southern Africa. 2000-2003 ICIPE Scientific Report. International Center for Insect Physiology and Ecology, Nairobi, Kenya.
- Kabissa JCB, (1989). Evaluation of damage thresholds for insecticidal control of I(Hübner) (Lepidoptera: Noctuidae) on cotton in eastern Tanzania. Bulletin of Entomological Research, 79(1):95-98.
- Kega, V. M. (2002). Research Status and Future Needs for Sustainable Management of the African Bollworm in Kwale District of Coastal Kenya. In Helicoverpa management in Kenya. Research Status and Needs. Proceedings of the KARI-ICIPE Workshop on Biocontrol-based IPM of the African Bollworm in Kenya. 16th November, Nairobi, Kenya. S. Sithanantham, C. Kairuki and J, Baya. (Editors). pp. 79-82.
- Kibata, G. N. (2002). The status of the African bollworm, Helicoverpa (Heliothis) armigera Hübner (Lepidoptera: Noctuidae) and future research needs for its management in Kenya. In 'Helicoverpa management in Kenya. Research Status and Needs. Proceedings of the KARI-ICIPE Workshop on Biocontrol Based IPM of the African Bollworm in Kenya. S. Sithanantham, C., Kariuki and J. Baya. (Editors). pp. 11-15.
- Magenya,O. E. V.(2002). Present knowledge and potential options for control of the African Bollworm in Southwestern Kenya. In Helicoverpa management in Kenya. Research Status and Needs. Proceedings of the KARI-ICIPE Workshop onBiocontrol-based IPM of the African Bollworm in Kenya. 16th November, Nairobi, Kenya. S. Sithanantham, C. Kairuki and J, Baya. (Editors). pp. 67-71.
- Matthews GA, Tunstall JP, (1968). Scouting for pests and the timing of spray applications. Cotton Growers' Review, 45:115-127
- NCIPM (2000).Annual report 1999-2000. National Centre for Integrated Pest Management, Indian Council for Agricultural Research, Lal Bahadur Shastri Building, Pusa Campur, New Delhi - 110 012, India
- Negash, T. and Abate, S. (2002). African bollworm management in the southern Region of Ethiopia. In African Bollworm Management in Ethiopia. Status and Needs. Proceedings of the National Workshop held at the Plant Protection Research Centre Ambo, Ethiopia. April 2002. pp 59-62.
- Nyambo, B. (1986). Studies in the bollworm Heliothis armigera Hübner, the key cotton pest in Tanzania, as a basis for improved integrated pest management. Thesis. Department of Pure and Applied Biology, Imperial College of Science and Technology. Silwood Park. U.K.
- Nyambo, B. (1990). Effect of natural enemies on the cotton bollworm Heliothis armigera Hübner (Lepidoptera: Noctuidae) in Western Tanzania. Tropical Pest Management. 36, 50-58.OISAT: Organisation for Non-Chemical Pest-Management in the Tropics www.oisat.org
- Pole, T. M. and Kimani, A. I. (2002). Research Status and Future Needs for Sustainable Management of the African Bollworm in Kilifi Region, Coastal Kenya. In 'Helicoverpa management in Kenya. Research Status and Needs'. Proceedings of the KARI-ICIPE Workshop on Biocontrol-based IPM of the African Bollworm in Kenya. 16th November, Nairobi, Kenya.S. Sithanantham, C. Kairuki and J, Baya. (Editors). pp. 75-78.
- Seif, A. A, Varela, A. M., Michalik S. and Löhr B. (2001): A Guide to IPM in French Beans Production with Emphasis on Kenya. ISBN: 92 9064 142 8.
- Sithanantham, S., Baumgartner, J. and Matoka, C. (2002). Ecosystem Approach for Management of Helicoverpa armiguera in Eastern Africa. In African Bollworm Management in Ethiopia. Status and needs. Proceedings of the National Workshop held at the Plant Protection Research Centre Ambo, Ethiopia. April 2002. pp 129-134.
- Stoll, G. (1986). Natural Crop Protection in the Tropics. AGRECOL. ISBN: 3-8236-1113-5.van den Berg, H. (1993). Natural control of Helicoverpa armigera in smallholder crops in East Africa. Thesis Wageningen.ISBN: 90-5485-107-4
- Varela, A. M., Seif, A. A. and Löhr B. (2003). A Guide to IPM in Tomato Production in Eastern and Southern Africa. ISBN: 92 9064 149 5
- Varela, A. M., and Seif, A. A. (2004). A Guide to IPM and Hygiene Standards in Okra Production in Kenya. ISBN: 92 9064 161 5
- Youdeowei, A. (2002). Integrated pest management practices for the production of vegetables. Ministry of Agriculture (MOFA) Plant Protection and Regulatory Services Directorate (PPRSD), Ghana, and the German Development Cooperation (GTZ). ISBN: 9988-0-1088-5.
